Introduction: AlloSCT is a potentially curative treatment for patients (pts) with AML. However, MRD persistence after transplantation has been identified as a risk factor for relapse. In this regard, the graft-versus-leukemia (GvL) effect is well recognized as one of the leading mechanisms of disease control after transplantation. Sabatolimab is a novel immunotherapy that targets the immuno-myeloid regulator TIM-3 on both immune cells and leukemic blasts, and may have the potential to enhance the GvL effect. STIMULUS-AML2 (NCT04623216) is a Phase Ib/II, open-label, multicenter study of sabatolimab alone or in combination with azacitidine as pre-emptive therapy in pts with AML who are in complete remission (CR) with positive MRD (MRD+) after alloSCT. Here we report preliminary data from the safety run-in of sabatolimab monotherapy at two dose levels (400 mg or 800 mg intravenous [IV] every four weeks [Q4W]).
Methods: Eligible pts for the safety run-in were adults (≥18 years [y]) with de novo or secondary AML, who achieved CR post-alloSCT but were MRD+ by local assessment at any time ≥Day (D) 60 after alloSCT (protocol amended from MRD+ D100-365 post alloSCT; March 24, 2022) and ≥2 weeks after immunosuppressive medications had been tapered off. MRD was assessed locally at baseline by polymerase chain reaction (PCR; 12 pts: NPM1 and/or WT1, 8; IDH2, 2; other, 2), multiparameter flow cytometry (MFC; 6 pts), both PCR and MFC (1 pt), and CD34+ chimerism (2 pts). In this safety run-in, adult pts received sabatolimab monotherapy at 400 mg or 800 mg IV on D1 of every 28-day cycle. The primary endpoint was incidence of treatment-emergent dose-limiting toxicity (DLT), including acute graft-vs-host disease (GvHD) and chronic GvHD during the first 2 cycles.
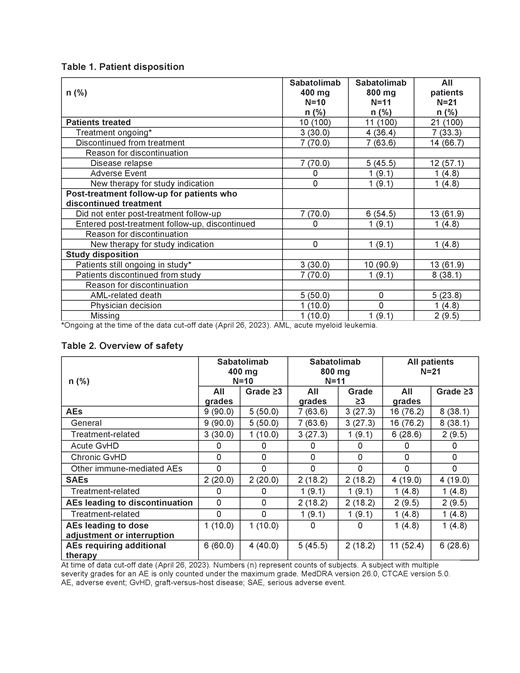
Results: A total of21 pts were enrolled in the safety run-in (10 at 400 mg; 11 at 800 mg). Median age was 59 years. At AML diagnosis, across cohorts, 8, 9 and 4 pts had favorable, intermediate, and adverse genetic risk (European LeukemiaNet 2017), respectively. At data cut-off (April 26, 2023), 3 pts were still ongoing in the 400 mg cohort and 7 had discontinued due to disease relapse. At 800 mg, 4 pts were ongoing and 7 had discontinued (5 due to relapse, 1 due to adverse event [AE], 1 due to new therapy) ( Table 1).
Across cohorts, there was 1 DLT of grade (G) 3 myocarditis after the first infusion of sabatolimab 800 mg, which recovered/resolved on the same day and sabatolimab was then withdrawn. An overview of safety is shown in Table 2. Any G and G≥3 AEs occurred in 16 (76.2%) and 8 (38.1%) pts, respectively. There were no cases of GvHD or immune-related AEs, 2 cases of G≥3 neutropenia and 1 case of G≥3 thrombocytopenia. G3 neutropenia and G3 thrombocytopenia in the same pt at 400 mg were considered treatment related and the patient later relapsed. At 400 mg, 2 pts experienced serious AEs (SAEs) after the DLT observation period (>3 cycles) and 2 pts at 800 mg had SAEs during the DLT period (G3 myocarditis [1 pt] and disease relapse with central nervous system involvement [1 pt]). There were 5 deaths, all at 400 mg and due to study indication (AML).
As a preliminary indicator of efficacy, 7 pts were still on treatment and in CR at data cut-off. At 400 mg, 2 pts and 1 pt had received 14 and 15 cycles of treatment (>1 y), respectively. At 800 mg, 1 pt had received 5, 1 pt 6, and 2 pts 7 cycles. At 400 mg, 7 pts relapsed: 2 pts after cycle 1, 2 pts after cycle 2, 1 pt each after cycles 3, 4 and 7. At 800 mg, 5 pts relapsed: 1 pt after cycle 1, 2 pts after cycle 2, 1 pt after cycle 3 and 1 pt after cycle 5.
Conclusions: In adult pts with AML who are in hematological CR with MRD+ after alloSCT, sabatolimab 400 mg and 800 mg monotherapies were well tolerated. Cytopenias occurred at low rates, were generally G1-2 and mostly related to imminent relapse. Importantly, there were no cases of GvHD reported or any immune-related AEs commonly seen with checkpoint inhibitors. Preliminary efficacy was promising with 30% of the pts at 400 mg still in CR after more than 1 y on treatment (available longer-term follow-up >1 y), and data may suggest a delayed onset of relapse. A dose expansion cohort at 800 mg opened for enrollment June 30, 2023. Adults will be randomized to sabatolimab as monotherapy or in combination with azacitidine, and preliminary efficacy on the prevention of hematological relapse will be evaluated. Adolescents (12-17 y) will also be enrolled to evaluate the safety of sabatolimab monotherapy.
This study is sponsored by Novartis.
Disclosures
Zeiser:Sanofi: Consultancy, Honoraria; MNK: Consultancy, Honoraria; incyte: Consultancy, Honoraria; novartis: Consultancy, Honoraria, Research Funding; Medac: Honoraria; VectivBio: Consultancy. Devillier:Astellas: Honoraria; Incyte: Honoraria; Jazz Pharmaceuticals: Honoraria; Medac: Honoraria; Sanofi: Honoraria; MSD: Honoraria. Mico':Novartis: Honoraria. Valcarcel:Takeda: Consultancy; Pfizer: Consultancy, Other: Travel expense reimbursement, Speakers Bureau; Jansen: Speakers Bureau; SOBI: Consultancy, Speakers Bureau; BMS/Celgene: Consultancy, Other: Travel expense reimbursement, Speakers Bureau; GSK: Consultancy, Other: Travel expense reimbursement; Amgen: Consultancy, Other: travel expense reimbursement, Speakers Bureau; Sanofi: Consultancy, Speakers Bureau; Jazz Pharmaceuticals: Consultancy, Other: Travel expense reimbursement, Speakers Bureau; Agios: Speakers Bureau; Novartis: Consultancy, Other: Travel expense reimbursement, Speakers Bureau; Astellas Pharma: Consultancy, Speakers Bureau; Kyte: Consultancy, Speakers Bureau; Gebro Pharma: Speakers Bureau; MSD: Consultancy, Speakers Bureau; Grifols: Speakers Bureau. Nourry:Novartis: Current Employment. Xu:Novartis: Current Employment. Medts:Novartis: Current Employment. Guichard:Novartis: Current Employment. Menssen:Novartis: Current Employment. Polverelli:BMS: Honoraria; GSK: Honoraria; Abbvie: Honoraria; Novartis: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal